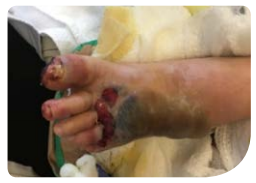
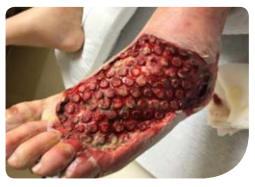
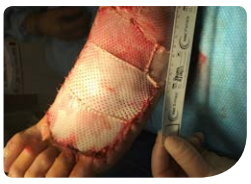
Review 1 (7 days from baseline):
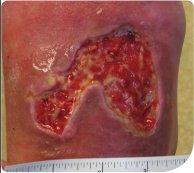
The wound size had reduced, measuring 5cm (length) by 5cm (width) by 2cm (depth), with sloughy tissue present on the bed of the ulcer (Figure 2). There was some strikethrough of exudate, but there was no leakage. The wound was prepared as before using surgical debridement, and PROMOGRAN™ Matrix was applied. Dressing changes were planned for twice a week.
Review 2 (14 days from baseline):
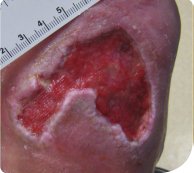
The wound had increased slightly in size, with slough (
40%) and granulating tissue (
60%) present in the wound bed (Figure 3). Serous exudate was at a moderate level. The wound bed was prepared as before, and the PROMOGRAN™ Matrix and non-border TIELLE™ ESSENTIAL Silicone Dressing regimen was continued.
Review 3 (21 days from baseline):
The wound measured 4.7cm (length) by 6.9cm (width) by 2cm (depth) (Figure 4), and there was a reduction in slough (
30% of the wound bed). There was a moderate level of serous exudate. Dressing regimen with PROMOGRAN™ Matrix and non-border TIELLE™ ESSENTIAL Silicone Dressing and offloading continued as before.
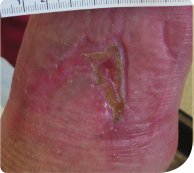
Review 4 (28 days from baseline):
The wound had reduced further to 3cm (length) by 3.2cm (width) by 2cm (depth), and the wound bed now comprised70% granulation tissue (Figure 5). Serous exudate levels remained moderate with strikethrough present during dressing wear time.
Final comments
Over 4 weeks, this DFU reduced in size by 50%, and there was an increase in granulating tissue. Throughout treatment, the clinician and patient were both highly satisfied with the dressings, with the patient feeling positive about the current treatment plan healing the wound. Though the wound had not completely healed, the use of PROMOGRAN™ Matrix with a secondary dressing of non-border TIELLE™ ESSENTIAL Silicone Dressing progressed the wound to a healing trajectory, and continued to be used beyond the 4-week evaluation period.
During the 4-week period, dressing changes reduced to twice a week. The patient rated the comfort of the PROMOGRAN™ Matrix and non-border TIELLE™ ESSENTIAL Silicone Dressing as ‘very good’. The patient began to feel more comfortable and less anxious, especially as her left limb had previously been amputated. All these improvements combined helped to improve the quality of life and outlook of the patient.
- Wounds International case studies evaluation. Using advanced wound
dressings in the local management of diabetic foot ulcers. London: Wounds International, 2018 (Suppl).