Case 3: V.A.C. VERAFLO CLEANSE CHOICE™ Dressing − Diabetic Foot
Author: Douglas Duke, DO Director of Wound Care Flowers Hospital, Dothan, AL
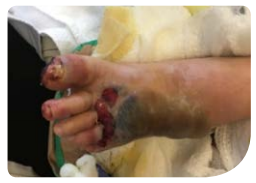
A 54-year-old male was admitted to the hospital with chronic left foot wound of unknown duration (Figure A). Patient comorbidities included hypertension, diabetes mellitus, and Charcot foot.
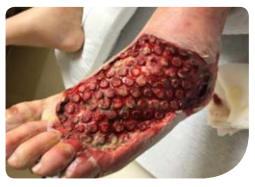
The patient was treated with an intravenous antibiotic regimen and underwent surgical debridement with excision of necrotic tissue (Figure B). Following debridement, the wound was managed with V.A.C. VERAFLO™ Therapy using V.A.C. VERAFLO CLEANSE CHOICE™ Dressing. The contact layer of the V.A.C. VERAFLO CLEANSE CHOICE™ Dressing was placed on the devitalized wound bed, followed by the cover layer. Fifty milliliters of a commercially available hypochlorous solution (Vashe® Wound Therapy Solution, SteadMed Medical, Fort Worth, TX) was instilled with a dwell time of 10
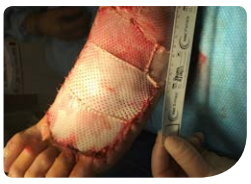
minutes, followed by 3.5 hours of continuous negative pressure at -125mmHg. After 2 days of V.A.C. VERAFLO™ Therapy, the V.A.C. VERAFLO CLEANSE CHOICE™ Dressing was changed (Figure C), and the wound bed displayed healthy granulation tissue with minimal devitalized tissue or thick slough. After 14 days and 4 dressing changes, therapy was discontinued. A human dermal collagen matrix (GRAFTJACKET™, Wright Medical Group, Memphis, TN) was then applied to the wound for closure (Figure D).
As with any case study, the results and outcomes should not be interpreted as a guarantee or warranty of similar results. Individual results may vary depending on the patient’s circumstances and condition.
NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for KCI products and therapies. Please consult a clinician and product instructions for use prior to application. This material is intended for healthcare professionals.
Copyright 2017, 2018 KCI Licensing, Inc. All rights reserved. Graftjacket is a trademark of Wright Medical Group. Unless otherwise designated, all trademarks are proprietary to KCI Licensing, Inc., its affiliates and/or licensors. PRA-PM-EU-00075 (08/18)